How saltwater can be converted into freshwater
Water is an essential part of a living organism, the human body weight may consist of up to 60% of water, without water living organisms cannot survive as water is a vital part of metabolism. About 71% of the earth’s surface is covered with water bodies and the oceans hold about 96% of the world’s water. About 96% of the water available on the earth is saline water, freshwater resources are limited to less than 4% of the available water on the earth and the resources are the river, lakes, groundwater, glaciers. The water in the river originates from the glaciers located in the mountain region and flows through the land and joins the sea. On the land, river water is used by humans, and the rest of the water flows into the sea. The water from the sea again returns to the earth through rainfall which is part of the water cycle. Due to the increasing population, the demand for freshwater is getting high as saline water/seawater is not fit for human use. Human has come with a various approach to meet the demand of freshwater and converting saltwater into freshwater is one among them.
Converting Saltwater into freshwater?
The technology used to achieve this is Reverse Osmosis. Reverse osmosis is a technology used to remove minute contaminants from water by making water pass under pressure through a semi-permeable membrane. The semipermeable membrane is a layer that acts like a filter that allows only particles that are in the size of water molecules to pass, the other unwanted particles are trapped by the membrane.
The semipermeable membrane is a biological membrane and is impermeable to large and polar molecules such as ions, proteins, and polysaccharides while being permeable to non-polar or hydrophobic molecules like lipids and small molecules of O2, CO2, N2, and NO.
Reverse osmosis is the reverse process of osmosis. Osmosis is a process in which the solvent molecule moves through a semipermeable membrane to the region of a higher concentration of the salute. The process of osmosis can occur naturally and easily but reverse osmosis requires external pressure called osmotic pressure and this phenomenon makes the pure solvent flow out of the solution through the semipermeable membrane and hence saltwater gets purified.
Reverse osmosis technology is used by water purifier companies like Bisleri, Aquafina for drinking water, beverage industries, pharmaceuticals.
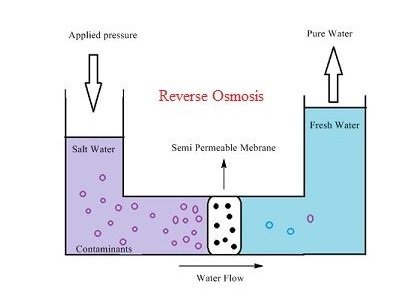
The purple water is saline water and the blue water is freshwater in the above diagram of the reverse osmosis and a semipermeable membrane is located in the center of the setup in such a way that when pressure is applied the water will pass through the membrane. The pressure is applied to the saline water column through some mechanism and pressure(osmotic pressure) is built up within the column, the solvent(water) will start passing through the membrane once the osmotic pressure crosses the limit and water will be moving towards freshwater column until the pressure is applied.


